A well-crafted survey consent form is the cornerstone of ethical and responsible research. It ensures participants understand the purpose of your study, their rights, and how their data will be handled. This understanding fosters trust and ultimately leads to more accurate and meaningful research results. Here’s how to create a survey consent form that meets both ethical standards and best practices.
 Example of a Survey Consent Form
Example of a Survey Consent Form
Understanding the Importance of a Comprehensive Survey Consent Form
A survey consent form, crucial for any research project involving human subjects, serves as a legal and ethical agreement between the researcher and the participant. It outlines the nature of the research, the participant’s role, and ensures informed participation. A poorly designed form can jeopardize your entire project, potentially leading to biased data and ethical concerns. citi human subjects research training can provide further insight into these ethical considerations.
Key Components of a Robust Consent Form
Every Survey Consent Form For Research must include several key elements. First, a clear and concise introduction explaining the study’s purpose and who is conducting it is essential. Second, the form should detail the procedures involved, including the estimated time commitment. Third, potential risks and benefits of participation must be clearly stated. Finally, the form should explicitly state how participant confidentiality will be maintained.
What information should be included in a survey consent form for research?
A comprehensive consent form needs to provide participants with all the information necessary for them to make an informed decision. This includes:
- Purpose of the Study: Explain why you’re conducting the survey and what you hope to achieve.
- Procedures: Describe what participants will be asked to do and how long it will take.
- Risks and Benefits: Be transparent about any potential risks or discomforts, however minimal, and any benefits to participants or society.
- Confidentiality: Explain how participant data will be stored and protected.
- Voluntary Participation: Clearly state that participation is voluntary and that participants can withdraw at any time without penalty.
- Contact Information: Provide contact information for the researcher and, if applicable, the Institutional Review Board (IRB).
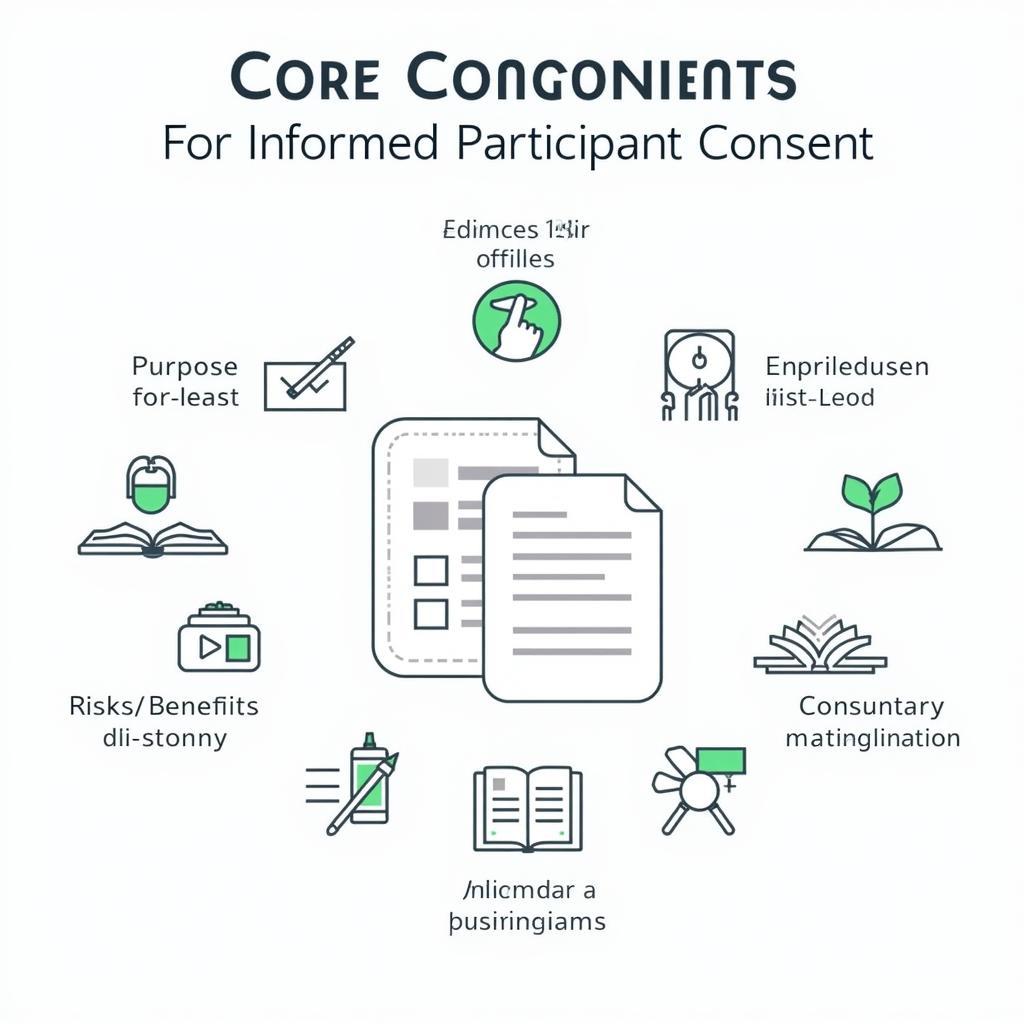 Essential Elements of a Survey Consent Form
Essential Elements of a Survey Consent Form
Are there different types of survey consent forms? Yes, the specific requirements for a survey consent form for research may vary depending on the research context, especially if dealing with sensitive topics or vulnerable populations. For example, research involving children or medical data requires additional safeguards. ib psychology research methods provides valuable resources for understanding these different research methodologies.
Best Practices for Creating User-Friendly Consent Forms
Even with all the necessary information, a poorly written consent form can be confusing and deter participation. To maximize clarity and comprehension:
- Use plain language: Avoid jargon and technical terms.
- Keep it concise: Get to the point without sacrificing clarity.
- Use clear headings and formatting: Break down the information into digestible chunks.
- Offer multiple formats: Consider providing the form in different languages or formats (e.g., audio, large print) if needed.
- Obtain proper consent: Ensure participants have ample opportunity to read and understand the form before agreeing.
“A well-designed consent form reflects not just ethical research practices but also respect for the participants,” says Dr. Amelia Reyes, a leading expert in research ethics. “It’s a crucial first step in building a strong researcher-participant relationship.”
Common Scenarios Requiring Survey Consent Forms
Survey consent forms are essential in a variety of research scenarios, including:
- Academic research: Studies conducted by universities or research institutions.
- Market research: Surveys gathering consumer opinions and preferences.
- Healthcare research: Studies involving patient data or medical procedures.
- Social science research: Surveys exploring social phenomena and human behavior. social science research is most likely to be challenged when ethical considerations are overlooked.
Conclusion: The Power of Informed Consent
A meticulously crafted survey consent form for research is indispensable for ethical and impactful research. It ensures that participants understand their rights and the research process, contributing to more robust and reliable findings. By following best practices and prioritizing transparency, you can build trust with participants and foster a positive research environment.
FAQ
- What is the purpose of a survey consent form?
- Who needs a survey consent form?
- What are the key elements of a consent form?
- What happens if I don’t use a consent form?
- Can a participant withdraw their consent after signing the form?
- Are there different types of consent forms for different research types?
- Where can I find templates for survey consent forms?
Other related articles on our website: paid research studies chicago and doctors research products.
Need help with your research? Contact us 24/7 at Phone Number: 0904826292, Email: research@gmail.com or visit us at No. 31, Alley 142/7, P. Phú Viên, Bồ Đề, Long Biên, Hà Nội, Việt Nam.