A Sponsor Research Agreement is the cornerstone of collaborative research endeavors, outlining the roles, responsibilities, and financial commitments of all parties involved. Whether you’re a seasoned researcher or just starting out, understanding the nuances of these agreements is crucial for a successful and mutually beneficial partnership. This article delves into the key aspects of sponsor research agreements, providing you with the insights needed to navigate this complex landscape.
A well-defined sponsor research agreement ensures a smooth research process, protects the intellectual property rights of all involved, and establishes clear communication channels. It’s essential for fostering trust and transparency, key ingredients for successful collaborations. We’ll cover everything from intellectual property ownership and publication rights to data sharing and confidentiality clauses, equipping you with the knowledge you need to confidently enter into research partnerships.
Just after beginning your exploration of sponsored research, it’s crucial to understand the legal framework. More than just a formality, the agreement acts as a roadmap, guiding the research journey and anticipating potential challenges.
Understanding the Components of a Sponsor Research Agreement
A typical sponsor research agreement comprises several key sections. These sections address specific aspects of the research collaboration, ensuring that all parties are on the same page. Let’s examine some of the most important components.
- Scope of Work: This section defines the precise research activities to be undertaken, including objectives, methodologies, and deliverables. A clearly defined scope minimizes misunderstandings and ensures everyone is working towards the same goals.
- Intellectual Property: This crucial component outlines ownership and usage rights of any inventions or discoveries resulting from the research. It’s vital for protecting the intellectual property of both the researcher and the sponsor.
- Budget and Payment Schedule: This section details the financial aspects of the agreement, specifying the total funding amount, payment milestones, and eligible expenses. Transparency in financial matters is crucial for a healthy sponsor-researcher relationship.
- Confidentiality: Research often involves sensitive data, making confidentiality clauses critical. These clauses protect proprietary information and prevent unauthorized disclosure.
- Publication Rights: This section clarifies who has the right to publish research findings and under what conditions. It also addresses authorship order and acknowledgment of the sponsor’s contribution. Understanding publication rights is especially important for researchers aiming to disseminate their work.
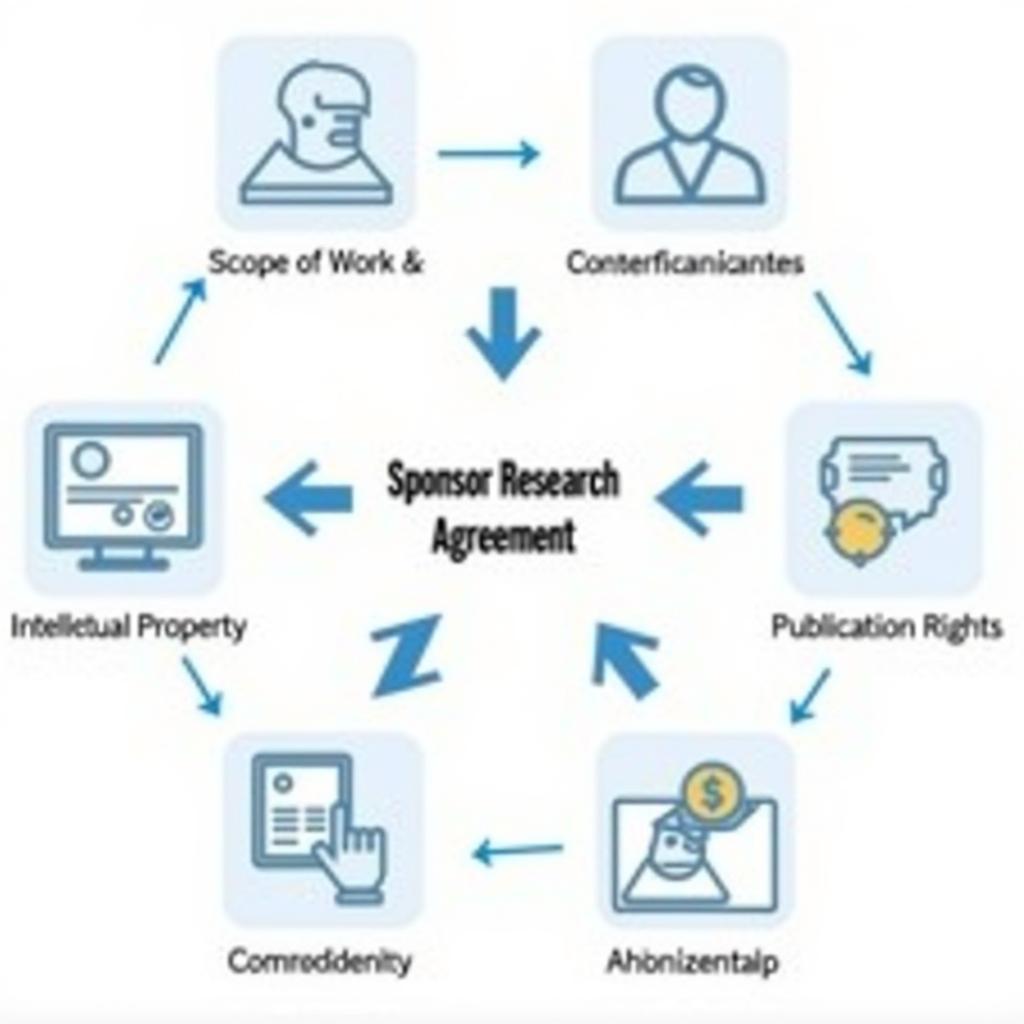 Key Components of a Sponsor Research Agreement
Key Components of a Sponsor Research Agreement
Negotiating a Sponsor Research Agreement
Negotiating a sponsor research agreement is a collaborative process. Both the researcher and the sponsor should approach the negotiation with open communication and a willingness to find mutually agreeable terms.
- Understand Your Needs: Before entering negotiations, clearly identify your own needs and priorities. This could include intellectual property ownership, funding requirements, or publication rights. Understanding your needs empowers you to advocate for your interests effectively.
- Seek Legal Advice: Consulting with an experienced attorney can help you navigate the legal complexities of the agreement. Legal counsel can provide invaluable insights and ensure that your rights are protected. This is especially helpful for those new to sponsored research.
- Be Prepared to Compromise: Negotiation often involves compromise. Be prepared to make concessions on certain points while holding firm on your core priorities.
- Document Everything: Keep detailed records of all communications and agreed-upon terms. This documentation can prove invaluable in case of future disputes.
Common Pitfalls to Avoid
While sponsor research agreements offer significant benefits, there are potential pitfalls to be aware of. By understanding these common challenges, you can take proactive steps to mitigate risks.
- Ambiguous Language: Vague wording can lead to misinterpretations and disputes. Ensure that all terms and conditions are clearly defined and easily understandable.
- Unrealistic Expectations: Setting unrealistic goals or timelines can create undue pressure and jeopardize the success of the research. Be realistic about what can be achieved within the given timeframe and resources.
- Ignoring Intellectual Property Rights: Failing to address intellectual property rights can lead to costly legal battles down the line. Clearly define ownership and usage rights from the outset.
For more information on research-related topics, explore our resources on sponsored research, federally funded research and development center and researcher pay rate. Understanding these aspects is crucial for navigating the research landscape effectively.
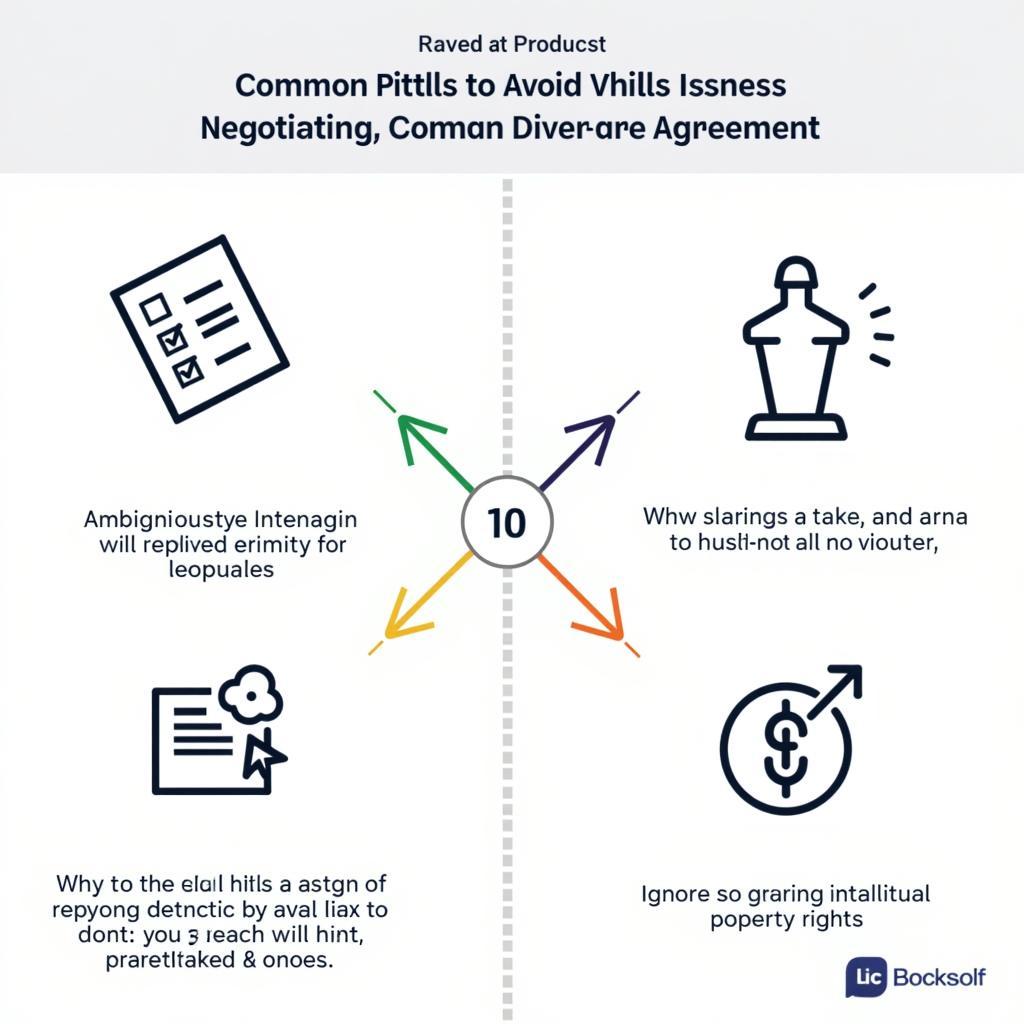 Common Pitfalls of Sponsor Research Agreements
Common Pitfalls of Sponsor Research Agreements
In conclusion, a sponsor research agreement is a vital document that governs collaborative research endeavors. Understanding its components, negotiating effectively, and avoiding common pitfalls are essential for a successful and mutually beneficial partnership. By carefully navigating the complexities of these agreements, researchers and sponsors can unlock the full potential of their collaboration and advance the frontiers of knowledge. Remember, a well-drafted sponsor research agreement sets the stage for a productive and rewarding research experience.
FAQ
- What is the typical duration of a sponsor research agreement? The duration varies depending on the nature and scope of the research project.
- Who owns the data generated during the research? The ownership of data is typically outlined in the agreement and can vary.
- What happens if a dispute arises between the sponsor and the researcher? The agreement should include a dispute resolution mechanism.
For further assistance, you can delve into related topics such as contract research organization clinical trials and form 1572 in clinical research. These resources provide valuable insights into specific areas of clinical research and related agreements.
Need support? Contact us 24/7 at 0904826292, email research@gmail.com, or visit No. 31, Alley 142/7, P. Phú Viên, Bồ Đề, Long Biên, Hà Nội, Việt Nam.