Medical research and clinical research are often used interchangeably, but they represent distinct stages in the process of advancing healthcare. This article explores the key differences between these two crucial areas of research, helping you understand their unique contributions to improving human health.  Medical Research vs. Clinical Research Overview
Medical Research vs. Clinical Research Overview
What is Medical Research?
Medical research encompasses a broad spectrum of scientific investigations aimed at improving our understanding of human health and disease. This can include basic science research conducted in laboratories, exploring the fundamental mechanisms of disease at a cellular or molecular level. It also includes pre-clinical research, such as animal studies, to test the safety and efficacy of new treatments before they are tested in humans. Medical research lays the groundwork for developing new therapies, diagnostic tools, and preventive strategies.
What are the different types of medical research? Several types exist, including basic research, applied research, and translational research. Basic research aims to expand our fundamental knowledge. Applied research uses that knowledge to develop practical solutions. Translational research bridges the gap between basic science and clinical applications, moving discoveries from the lab to the patient’s bedside. basic science vs clinical research
What is Clinical Research?
Clinical research specifically focuses on studying the safety and effectiveness of interventions in humans. This type of research involves human participants and is conducted in various settings, including hospitals, clinics, and research centers. Clinical trials are a hallmark of clinical research, evaluating new drugs, medical devices, or procedures. Clinical research plays a vital role in determining whether a new treatment is safe and effective for widespread use.
What are the phases of clinical research? Clinical trials typically progress through four phases, each with specific objectives. Phase I trials assess the safety of a new intervention in a small group of healthy volunteers. Phase II trials examine the efficacy of the intervention in a larger group of patients with the target condition. Phase III trials compare the new intervention to existing treatments in a much larger patient population. Finally, Phase IV trials monitor the long-term effects and safety of the intervention after it has been approved for general use.
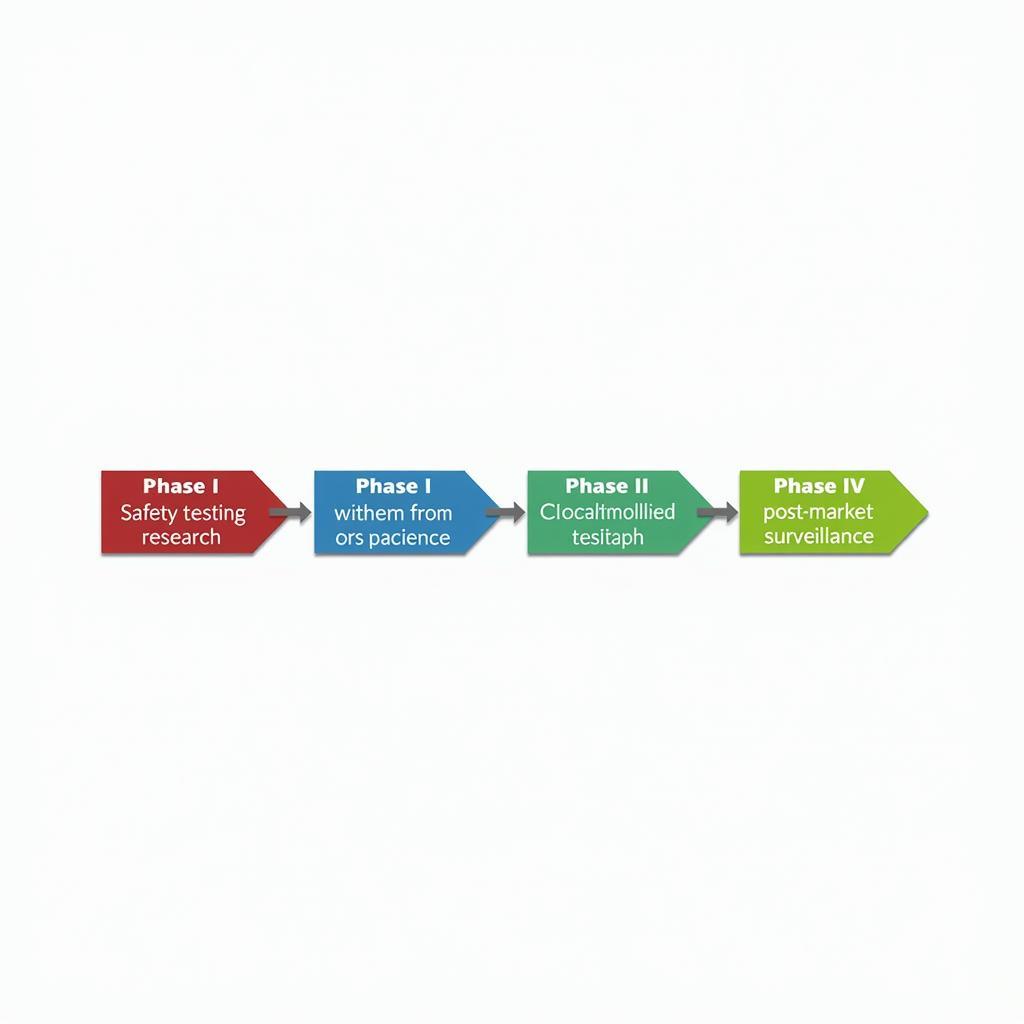 Clinical Research Phases Explained
Clinical Research Phases Explained
Key Differences Between Medical Research vs. Clinical Research
One of the fundamental differences between medical research and clinical research lies in their scope. Medical research is broader, encompassing a wider range of scientific investigations. Clinical research, on the other hand, is a subset of medical research specifically focused on human participants and clinical interventions.
Methodologically, medical research employs diverse techniques, including laboratory experiments, animal studies, and computational modeling. Clinical research primarily utilizes controlled clinical trials to evaluate the safety and efficacy of treatments.
“Medical research is the foundation upon which clinical research is built,” explains Dr. Amelia Carter, a renowned research scientist. “Without the discoveries made in the lab, there would be no new treatments to test in clinical trials.”
pharmaceutical research associates often work on the medical research side, developing new compounds and therapies. Their work is essential for providing the basis for future clinical trials.
 Medical and Clinical Research Collaboration
Medical and Clinical Research Collaboration
The Importance of Both Medical and Clinical Research
Both medical and clinical research are essential for advancing healthcare. Medical research provides the fundamental knowledge and tools necessary for developing new treatments. Clinical research then rigorously tests these treatments in humans, ensuring their safety and effectiveness before they become widely available. market research healthcare plays a crucial role in understanding the needs and preferences of patients, which can inform both medical and clinical research efforts.
“Clinical research allows us to translate scientific discoveries into real-world benefits for patients,” adds Dr. David Lee, a leading clinical trial investigator. “It’s a critical step in bringing new and improved therapies to those who need them most.” Specific populations, such as pregnant women, require careful consideration in research studies. research studies for pregnant women are crucial for understanding how medications and treatments affect both the mother and the developing fetus.
Conclusion
Medical research and clinical research are distinct yet interconnected fields that contribute significantly to improving human health. Understanding their differences is crucial for appreciating the complex process of translating scientific discoveries into effective treatments and interventions. By supporting both medical and clinical research, we can pave the way for a healthier future. clinical research coordinator vs associate highlights the different roles within clinical research teams, showcasing the collaborative efforts needed to conduct successful clinical trials.
Need support? Contact us 24/7: Phone: 0904826292, Email: [email protected], or visit us at No. 31, Alley 142/7, P. Phú Viên, Bồ Đề, Long Biên, Hà Nội, Việt Nam.