Informed consent is the bedrock of ethical research. Understanding what a consent form entails, and seeing an Example Of A Consent Form For Research Study, is crucial for both researchers and participants. A well-drafted consent form ensures participants understand the study’s purpose, procedures, risks, and benefits, empowering them to make an informed decision about their involvement.
Research involving human subjects requires careful consideration of ethical principles. One such principle is the requirement for informed consent. This involves providing potential participants with comprehensive information about the study and obtaining their voluntary agreement to participate. Having a clear example of a consent form for research study can be incredibly helpful. Let’s delve into the essential elements of a well-structured consent form.
Key Elements of a Consent Form
A robust consent form should cover several key areas to ensure participant understanding and protection. These elements include:
-
Purpose of the Research: Clearly explain the study’s aims and objectives in plain language, avoiding jargon. What questions are you trying to answer?
-
Study Procedures: Detail what participants will be expected to do, including the time commitment, types of activities, and any potential discomfort. Be specific and transparent.
-
Risks and Benefits: Honestly assess and disclose potential risks, both physical and psychological. Also, outline potential benefits to participants or society. Honesty is paramount.
-
Confidentiality: Explain how participant data will be protected and stored. Will data be anonymized? Who will have access to it?
-
Voluntary Participation: Emphasize that participation is entirely voluntary and that participants can withdraw at any time without penalty.
-
Contact Information: Provide contact information for the research team and any relevant oversight bodies, such as institutional review boards (IRBs).
Just after the introduction, we encourage you to explore more about research questions, specifically on topics such as criminal justice, by visiting our research questions on criminal justice page.
Example Consent Form Structure
Below is a simplified example of a consent form for research study. Remember, this is a template and should be adapted to fit the specific requirements of each individual study.
Heading and Introduction
The form should begin with a clear title, such as “Informed Consent Form,” followed by a brief introduction to the study and the research team.
Study Information
This section should provide details about the purpose, procedures, duration, and location of the study.
Risks and Benefits
Clearly outline any potential risks and benefits associated with participation.
Confidentiality
Explain how participant data will be handled and protected.
Voluntary Participation and Withdrawal
Emphasize the voluntary nature of participation and the right to withdraw at any time.
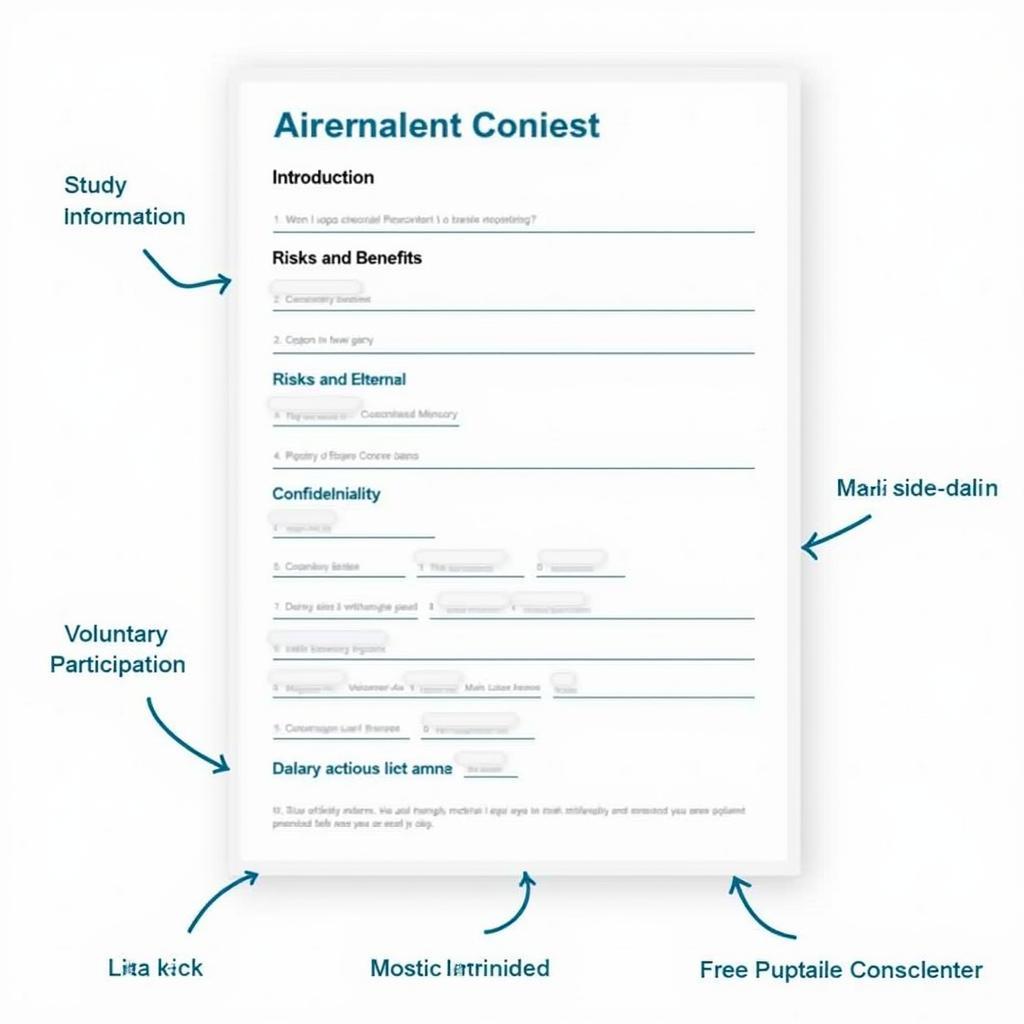 Example Consent Form Structure
Example Consent Form Structure
Contact Information
Provide contact details for the research team and IRB.
Signature Lines
Include spaces for both the participant and a member of the research team to sign and date the form.
Understanding the practice of social research can further enhance your grasp of the ethical considerations involved in studies. Our resource on the practice of social research offers valuable insights into this crucial aspect.
Importance of a Well-Written Consent Form
A well-written consent form is not just a legal requirement; it is a fundamental ethical tool. It fosters trust between researchers and participants, promotes transparency, and ensures that individuals are empowered to make informed decisions about their involvement in research. A clear example of a consent form for research study serves as a valuable guide in this process.
What are some common questions about consent forms?
What if a participant doesn’t understand the consent form? Researchers should be prepared to answer questions and explain the information in a clear and accessible way. Consider providing the form in multiple languages if necessary.
Can a consent form be changed after it has been signed? Amendments to a consent form require IRB approval and must be communicated clearly to participants, who must then re-consent.
Is a verbal consent ever acceptable? While written consent is generally preferred, verbal consent may be acceptable in certain circumstances, with appropriate documentation and IRB approval.
Delving into specific research methods can provide a more comprehensive understanding of consent procedures. You might find our resource on research methods in criminology and criminal justice helpful in this regard.
Conclusion
A comprehensive and easily understandable example of a consent form for research study is essential for conducting ethical research. By ensuring participants are fully informed about the study’s purpose, procedures, risks, and benefits, researchers promote transparency, build trust, and empower individuals to make autonomous decisions about their participation. Remember to tailor your consent form to your specific study and always prioritize the rights and well-being of your participants.
 Conclusion: The Importance of Consent Forms in Research
Conclusion: The Importance of Consent Forms in Research
FAQs
- What is the purpose of a consent form?
- Who needs a consent form?
- What information should be included in a consent form?
- Can a minor sign a consent form?
- What happens if a participant withdraws from a study?
Exploring related research questions can be enlightening. For instance, consider our page on psychological research questions or our article on linear clinical research.
If you need support, please contact us at Phone: 0904826292, Email: research@gmail.com, or visit us at No. 31, Alley 142/7, P. Phú Viên, Bồ Đề, Long Biên, Hà Nội, Việt Nam. Our customer service team is available 24/7.