Empire Clinical Research plays a crucial role in advancing medical knowledge and developing new treatments for a wide range of diseases. This intricate process, often shrouded in mystery, involves rigorous scientific investigation and careful observation of human participants. Understanding the intricacies of empire clinical research is paramount for both researchers and potential participants.
What exactly is empire clinical research? It’s a structured and controlled process that follows strict ethical guidelines to evaluate the safety and effectiveness of new medical interventions. These interventions can include drugs, medical devices, diagnostic tests, and even behavioral therapies. The overarching goal is to generate robust scientific evidence that informs medical practice and ultimately improves patient outcomes. See our article on clinical research la for more insights on clinical research in Louisiana.
Delving into the Stages of Empire Clinical Research
Clinical research typically progresses through a series of distinct phases, each with its own specific objectives and procedures.
Phase 1: Initial Safety Testing
This initial phase focuses primarily on assessing the safety profile of a new intervention in a small group of healthy volunteers. Researchers meticulously monitor participants for any adverse effects and gather preliminary data on the intervention’s pharmacokinetics (how the body processes the intervention) and pharmacodynamics (how the intervention affects the body).
Phase 2: Evaluating Effectiveness
Once deemed safe enough to proceed, the intervention moves into Phase 2, where its effectiveness is evaluated in a larger group of participants with the targeted condition. This phase aims to establish whether the intervention demonstrates the desired therapeutic effect and to identify the optimal dosage regimen.
Phase 3: Confirming Effectiveness and Safety
Phase 3 involves a substantially larger and more diverse group of participants to confirm the intervention’s effectiveness, monitor side effects, compare it to existing treatments, and gather information that will allow the intervention to be used safely.
Phase 4: Post-Market Surveillance
Even after an intervention is approved and available to the public, research continues in Phase 4, also known as post-market surveillance. This phase aims to monitor long-term effects, identify rare side effects, and refine treatment strategies.
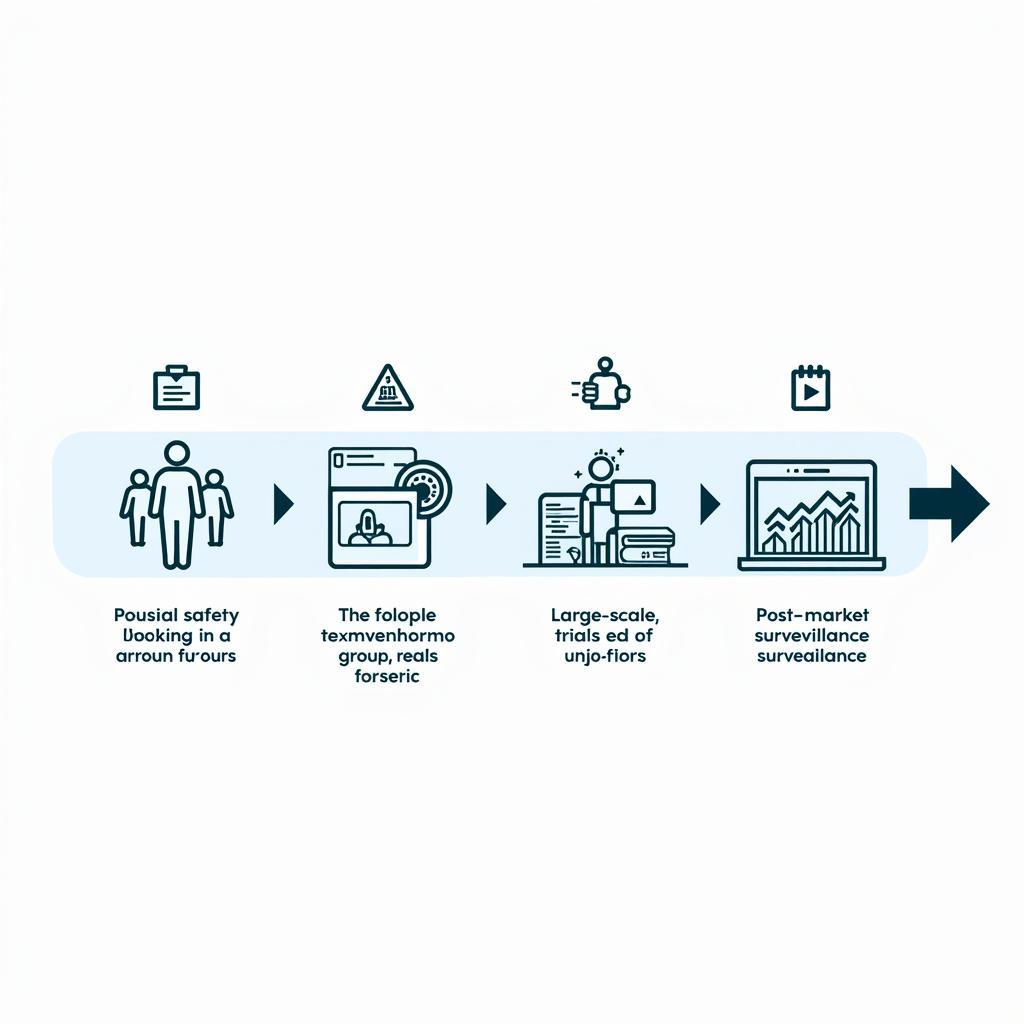 Empire Clinical Research Phases
Empire Clinical Research Phases
The Importance of Ethical Considerations in Empire Clinical Research
Ethical considerations are paramount throughout the entire clinical research process. Protecting the rights, safety, and well-being of participants is of utmost importance. Informed consent is a cornerstone of ethical clinical research, ensuring that participants understand the risks and benefits involved before voluntarily agreeing to participate.
Ethical review boards, composed of experts and community representatives, meticulously review research protocols to ensure they adhere to ethical principles and regulatory guidelines. These boards play a vital role in safeguarding the interests of research participants. You can find more information on clinical research at empire clinical research upland.
What are the benefits of participating in empire clinical research?
Participating in clinical research can offer several potential benefits, including access to innovative treatments, contributing to medical advancements, and close monitoring by medical professionals. However, it’s essential to carefully weigh the potential risks and benefits before making a decision.
How to Find Clinical Trials related to Empire Clinical Research?
Numerous resources are available to help individuals find clinical trials that align with their health conditions and interests. Online databases, such as ClinicalTrials.gov, provide comprehensive information about ongoing clinical research studies. Consulting with healthcare professionals can also be valuable in identifying relevant clinical trials. Explore more about research in jedi survivor research tanalorr.
Conclusion: The Future of Empire Clinical Research
Empire clinical research holds immense promise for improving human health and tackling some of the most challenging medical problems. Continued advancements in research methodologies, coupled with a strong commitment to ethical principles, will pave the way for even more effective and personalized treatments in the future. Empire clinical research remains a beacon of hope for patients and a testament to the power of scientific inquiry.
FAQ
- What is the difference between Phase 2 and Phase 3 clinical trials?
- What are the ethical considerations in clinical research?
- How can I find clinical trials near me?
- What are the risks of participating in a clinical trial?
- What is informed consent in the context of clinical research?
- How are clinical trials regulated?
- What is the role of an Institutional Review Board (IRB)?
For further research insights, see finance research letters.
If you need assistance, please contact us at Phone Number: 0904826292, Email: research@gmail.com, or visit our address at No. 31, Alley 142/7, P. Phú Viên, Bồ Đề, Long Biên, Hà Nội, Việt Nam. We have a 24/7 customer service team.