CAPA, or Corrective and Preventive Action, is a crucial process in clinical research. It ensures the integrity and reliability of research data, promoting patient safety and regulatory compliance. This article delves into the significance of Capa In Clinical Research, exploring its various aspects and benefits. capa process in clinical research
Understanding the Importance of CAPA
CAPA is a systematic approach to identifying and addressing issues that arise during clinical trials. These issues, ranging from minor deviations to critical non-compliance, can compromise the quality and validity of the research findings. By implementing a robust CAPA system, research organizations can proactively mitigate risks, prevent recurrence of problems, and ensure adherence to Good Clinical Practice (GCP) guidelines. Effectively managing CAPA can be essential for ensuring accurate and reliable results in a clinical research setting.
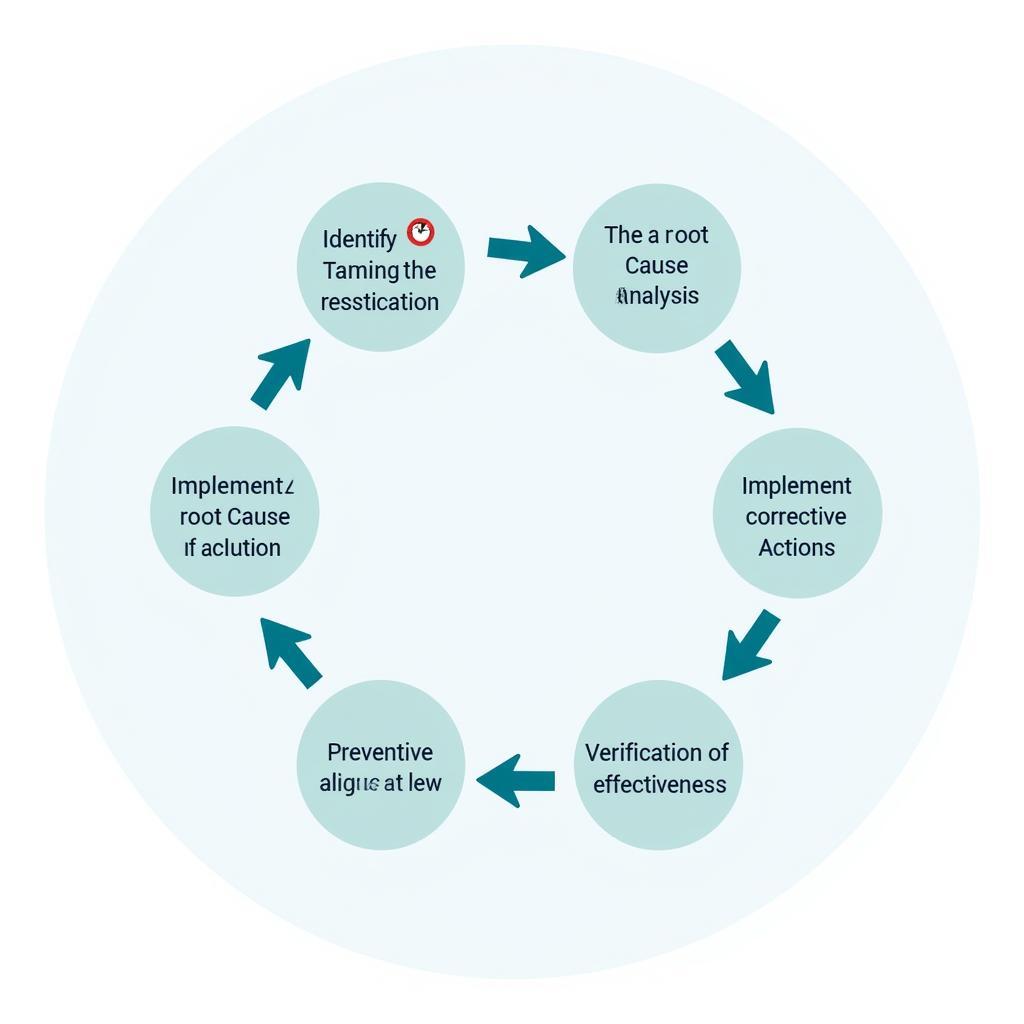 CAPA Process Flowchart
CAPA Process Flowchart
Implementing an Effective CAPA System
Establishing a well-defined CAPA system involves several key steps. First, a clear procedure for reporting and documenting deviations and non-conformities should be in place. This includes defining responsibilities, timelines, and communication channels. Second, a thorough investigation process is crucial to understand the root cause of the issue. This often involves analyzing data, interviewing personnel, and reviewing relevant documentation. For researchers looking for facilities nearby, convenient access to market research facilities near me can streamline the CAPA process and ensure adherence to regulatory requirements.
Key Steps in the CAPA Process
- Identification: Recognize and document the problem.
- Investigation: Conduct a thorough investigation to determine the root cause.
- Corrective Action: Implement actions to address the immediate issue and prevent recurrence.
- Preventive Action: Implement actions to prevent similar problems from occurring in the future.
- Verification: Confirm the effectiveness of the implemented actions.
More information on clinical research processes can be found at capa clinical research. Understanding the intricacies of database research can also be valuable. You can explore more about what is database research.
Benefits of CAPA in Clinical Research
Implementing a robust CAPA system brings numerous benefits to clinical research. It improves data quality and reliability by addressing and preventing errors. It enhances patient safety by minimizing risks associated with deviations from protocol. It strengthens regulatory compliance by ensuring adherence to GCP guidelines. This dedicated space specifically designed for medical research can significantly enhance the implementation of robust CAPA systems. Read more on medical research building iii. A well-structured CAPA system can save valuable time and resources by proactively addressing potential issues.
“CAPA is not just about fixing problems; it’s about continuous improvement and building a culture of quality in clinical research,” says Dr. Emily Carter, a leading expert in clinical trial management. “By embracing CAPA, research organizations demonstrate their commitment to patient safety and the integrity of their research.”
Conclusion
CAPA is an indispensable component of clinical research, ensuring data integrity, patient safety, and regulatory compliance. By implementing a robust and well-defined CAPA system, research organizations can proactively mitigate risks, enhance the quality of their research, and contribute to the advancement of medical knowledge. Addressing issues effectively through CAPA is key to achieving reliable and trustworthy results in clinical research.
FAQ
- What does CAPA stand for?
- Why is CAPA important in clinical research?
- What are the key steps in the CAPA process?
- What are the benefits of implementing a CAPA system?
- Who is responsible for CAPA in a clinical trial?
- How often should CAPA be reviewed?
- What are some common examples of CAPAs in clinical research?
For further assistance, please contact us at Phone Number: 0904826292, Email: research@gmail.com or visit our address at No. 31, Alley 142/7, P. Phú Viên, Bồ Đề, Long Biên, Hà Nội, Việt Nam. We have a 24/7 customer support team.