Alcoa Research Acronym, a cornerstone of data integrity, stands for Attributable, Legible, Contemporaneous, Original, and Accurate. This principle guides researchers, especially in regulated fields like pharmaceuticals and healthcare, to ensure the reliability and trustworthiness of their data. Understanding and applying ALCOA principles is crucial for maintaining scientific rigor and making sound decisions based on credible evidence.
What Does ALCOA Research Acronym Stand For?
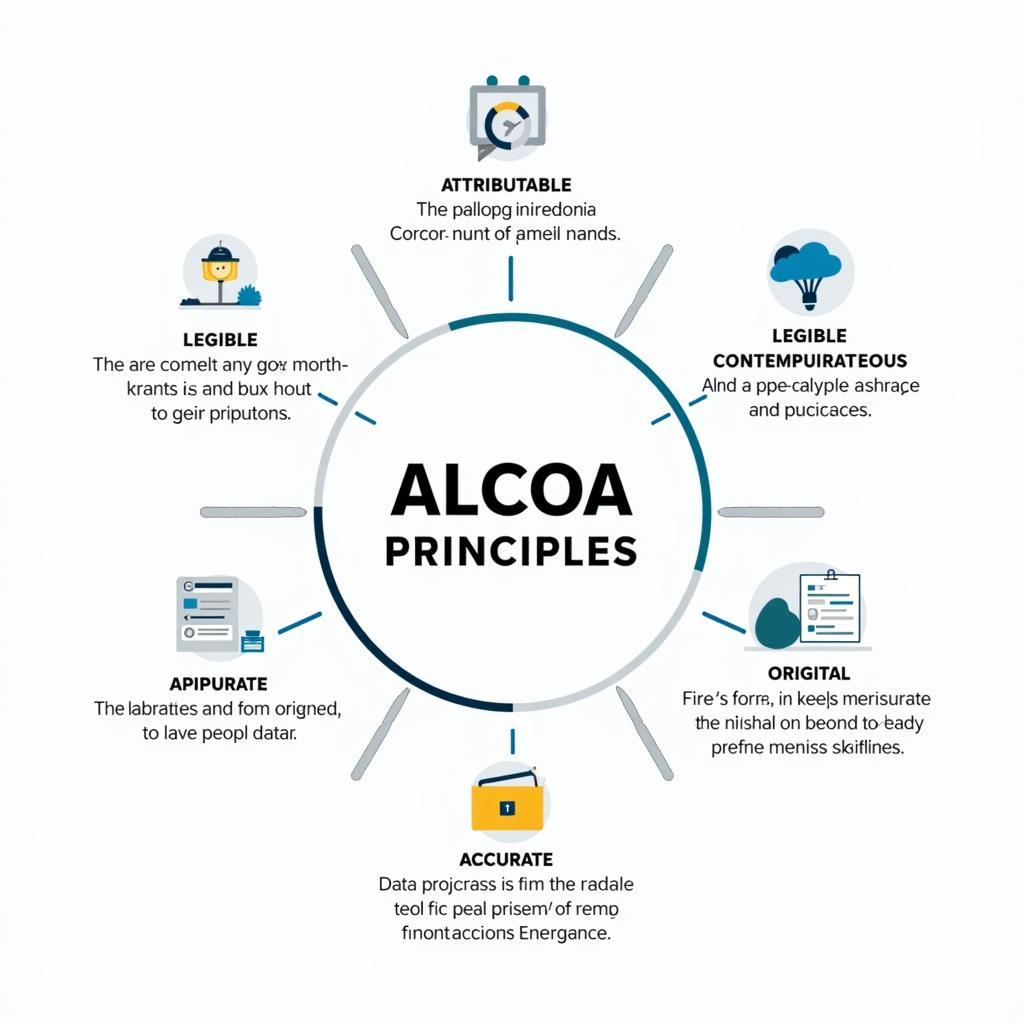
The ALCOA research acronym provides a framework for ensuring data quality. Each letter represents a key characteristic that data must possess to be considered reliable. Let’s break down each component:
- Attributable: Knowing who acquired the data and when is essential. This ensures accountability and allows for verification if needed. Imagine a paranormal investigation where evidence is found. Knowing who discovered it, their experience level, and the exact time is crucial for proper assessment.
- Legible: Data should be permanent and easily readable. Scribbled notes on a napkin won’t cut it. Think of ancient texts – their legibility (or lack thereof) directly impacts our ability to understand past civilizations.
- Contemporaneous: Data should be recorded at the time it is generated. This prevents memory bias and ensures accuracy. Like documenting a UFO sighting immediately, rather than relying on recollections days later.
- Original: The first recorded data is the most valuable. Preserving the original records, whether physical or electronic, is paramount. In paranormal research, this could be the original audio recording of an EVP session, not a copy.
- Accurate: Data must be error-free and reflect the true observation or result. Accuracy is the foundation of reliable conclusions. Imagine measuring EMF fluctuations during a ghost hunt; accurate readings are vital for meaningful analysis.
 ALCOA Principles Diagram
ALCOA Principles Diagram
Why is the ALCOA Research Acronym Important?
The ALCOA research acronym is crucial for maintaining data integrity, especially in fields where decisions based on data can have significant consequences. In pharmaceutical research, for example, adhering to ALCOA ensures the safety and efficacy of new drugs. In Paranormal Research, it helps us distinguish between genuine phenomena and flawed data.
“ALCOA principles are not just guidelines, they are the bedrock of trustworthy research,” says Dr. Emily Carter, a leading researcher in data integrity. “Without adherence to these principles, the validity of any findings is questionable.”
Expanding ALCOA: ALCOA+
In recent years, the ALCOA acronym has been expanded to ALCOA+ to further enhance data integrity. The “+” incorporates several additional principles:
- Complete: No missing information.
- Consistent: Data is consistent across all records.
- Enduring: Data is accessible for the required period.
- Available: Data is readily available for review or inspection.
These additions build upon the foundation of ALCOA, creating a more robust framework for ensuring data quality in the digital age. For instance, in a paranormal investigation, ensuring complete and consistent data across multiple investigators’ logs strengthens the overall credibility of the findings.
“ALCOA+ addresses the challenges of data management in the modern research environment,” explains Dr. Michael Davies, an expert in digital data preservation. “These additions ensure data remains accessible and usable throughout its lifecycle.”
ALCOA Research Acronym: Practical Applications
Applying ALCOA principles in real-world scenarios can significantly enhance the reliability of research. Consider the following examples:
- Laboratory Notebooks: Using bound notebooks, writing in permanent ink, and dating each entry are essential for maintaining attributable, legible, and contemporaneous records.
- Electronic Data Capture: Validated software systems with audit trails ensure data integrity by automatically capturing who made changes and when.
 Electronic Data Capture System in Research
Electronic Data Capture System in Research
Conclusion
The ALCOA research acronym provides a vital framework for ensuring data integrity in various fields, from pharmaceutical research to paranormal investigations. By adhering to these principles, researchers can ensure their data is attributable, legible, contemporaneous, original, and accurate. ALCOA+ further strengthens this framework by emphasizing completeness, consistency, endurance, and availability. Ultimately, embracing ALCOA principles enhances the credibility and trustworthiness of research findings.
FAQ
- What does the “O” in ALCOA stand for? Original.
- Why is ALCOA important for research? It ensures data integrity and reliability.
- What is ALCOA+? An expanded version of ALCOA with additional principles.
- How can I apply ALCOA in my research? By maintaining detailed records, using validated systems, and following best practices for data management.
- What are the benefits of using ALCOA? Increased credibility and trustworthiness of research findings.
- Is ALCOA applicable to all types of research? Yes, it is relevant across various disciplines.
- Where can I learn more about ALCOA? Numerous resources are available online and in research publications.
Need assistance with your research? Contact us! Phone: 0904826292, Email: research@gmail.com. Visit us at: No. 31, Alley 142/7, P. Phú Viên, Bồ Đề, Long Biên, Hà Nội, Việt Nam. We have a 24/7 customer support team.