Starting a clinical research site can be a rewarding but complex endeavor. It requires careful planning, significant financial investment, and a deep understanding of the clinical research process. This guide will provide a comprehensive overview of How To Start A Clinical Research Site, covering everything from developing a business plan to recruiting and managing patients.
Developing a Solid Business Plan
 Developing a Clinical Research Business Plan
Developing a Clinical Research Business Plan
A well-structured business plan is crucial for attracting investors and securing funding. It should outline your site’s mission, target patient population, therapeutic areas of focus, and projected financial outlook. A SWOT analysis, identifying your site’s Strengths, Weaknesses, Opportunities, and Threats, can provide valuable insights for strategic decision-making.
Building a Qualified Team
 Assembling a Highly Qualified Clinical Research Team
Assembling a Highly Qualified Clinical Research Team
The success of your clinical research site hinges on a skilled and experienced team. Key personnel includes:
- Principal Investigator (PI): A licensed physician with extensive research experience, responsible for the scientific integrity of the trials.
- Clinical Research Coordinators (CRCs): Responsible for managing the day-to-day operations of clinical trials, including patient recruitment, data collection, and regulatory compliance.
- Research Assistants: Provide support to the CRCs in various tasks.
Establishing Essential Infrastructure
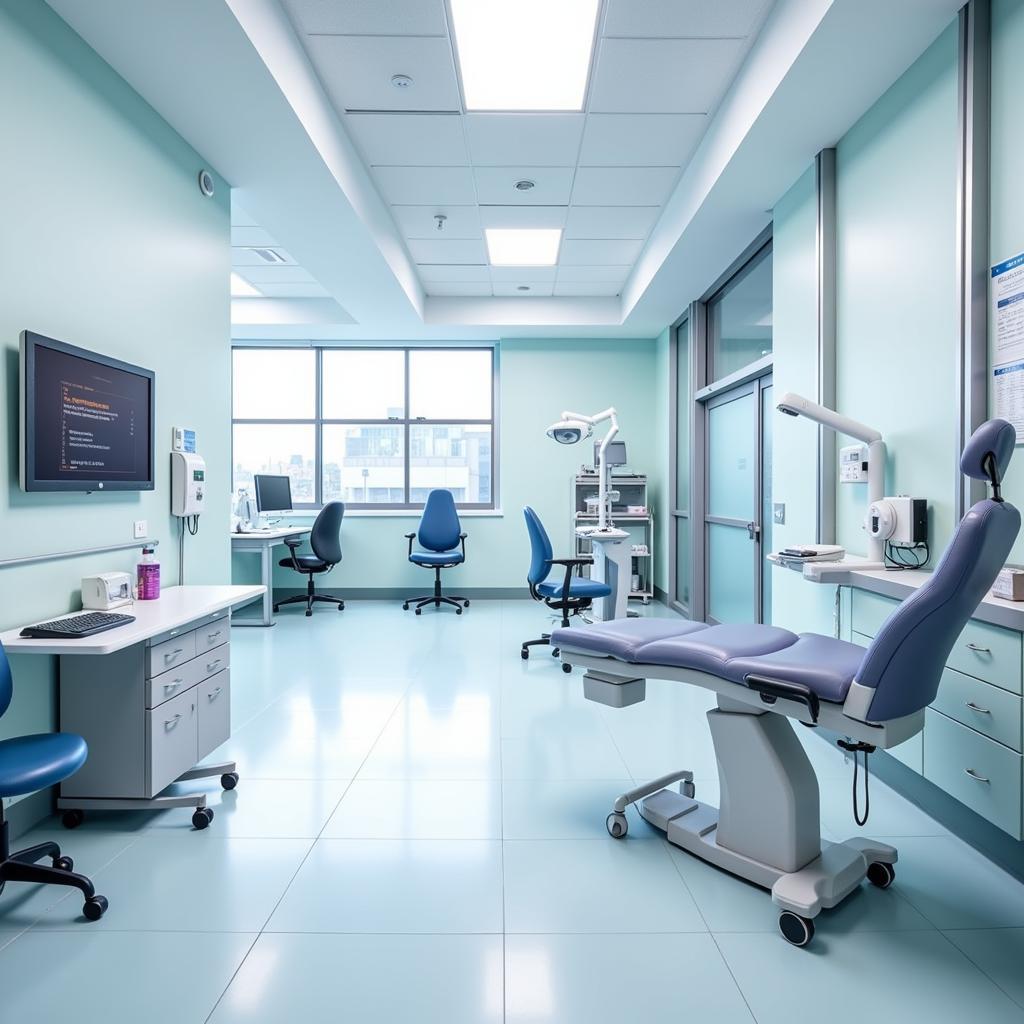 Setting Up a State-of-the-Art Clinical Research Facility
Setting Up a State-of-the-Art Clinical Research Facility
Your facility needs to meet strict regulatory standards and patient comfort requirements. This includes:
- Dedicated space: Examination rooms, a laboratory, and secure data storage.
- Equipment: Medical devices, centrifuges, freezers for sample storage, and computers.
- Software: Electronic Data Capture (EDC) systems for efficient data management.
Obtaining Necessary Licenses and Certifications
Operating a clinical research site requires obtaining licenses and certifications from various regulatory bodies, including:
- State Medical Board: Ensure all medical professionals are licensed to practice in your state.
- Institutional Review Board (IRB): Responsible for overseeing the ethical conduct of research involving human subjects.
- Food and Drug Administration (FDA): May be required depending on the type of research you conduct.
Navigating the Financial Aspects
Starting a clinical research site requires substantial financial investment. You’ll need to secure funding to cover:
- Startup costs: Facility setup, equipment purchase, and staff hiring.
- Operational expenses: Salaries, rent, utilities, and trial-related costs.
Effective Patient Recruitment and Retention
Patient recruitment and retention are vital to the success of clinical trials. Implement a multi-pronged approach that includes:
- Community outreach: Attending health fairs, partnering with local organizations, and raising awareness through social media.
- Physician referrals: Building relationships with physicians who can refer eligible patients.
- Patient databases: Utilizing databases to identify potential participants.
Ensuring Compliance and Quality Control
Maintaining rigorous compliance with regulatory guidelines and ethical standards is paramount. Establish robust Standard Operating Procedures (SOPs) for all aspects of your operations.
Conclusion
Starting a clinical research site demands meticulous planning, dedication, and a commitment to ethical research practices. By following the steps outlined in this guide and adapting them to your unique circumstances, you can increase your chances of establishing a successful and reputable clinical research site.
FAQs about Starting a Clinical Research Site
1. What is the average cost of starting a clinical research site?
The startup cost for a clinical research site can vary significantly but typically ranges from $500,000 to over $1 million, depending on factors like location, size, and equipment needs.
2. How long does it take to get a clinical research site up and running?
It can take anywhere from six months to a year or longer to establish a fully operational clinical research site.
3. What are the biggest challenges of running a clinical research site?
Patient recruitment, maintaining financial stability, and managing the regulatory burden are among the most significant challenges.
4. How can I attract pharmaceutical companies to sponsor trials at my site?
Building a strong reputation for quality data, patient recruitment success, and efficient trial management is crucial.
5. What are some resources available to help me start a clinical research site?
Organizations like the Association of Clinical Research Professionals (ACRP) and the Society for Clinical Research Sites (SCRS) offer valuable resources, education, and networking opportunities.
For more detailed information on clinical research careers and related topics, please visit:
- sr research scientist salary
- clinical research management degree
- research jobs minneapolis
- clinical research associate medpace
- best certifications for clinical research
Need assistance starting your clinical research site journey? We’re here to help! Contact us at Phone Number: 0904826292, Email: research@gmail.com or visit us at No. 31, Alley 142/7, P. Phú Viên, Bồ Đề, Long Biên, Hà Nội, Việt Nam. Our dedicated customer support team is available 24/7 to answer your questions and guide you through the process.