Clinical Research Risk Management is crucial for ensuring patient safety, data integrity, and the overall success of a clinical trial. It involves identifying, assessing, mitigating, and monitoring potential risks throughout the research process. Effective risk management requires a proactive approach, meticulous planning, and continuous evaluation. 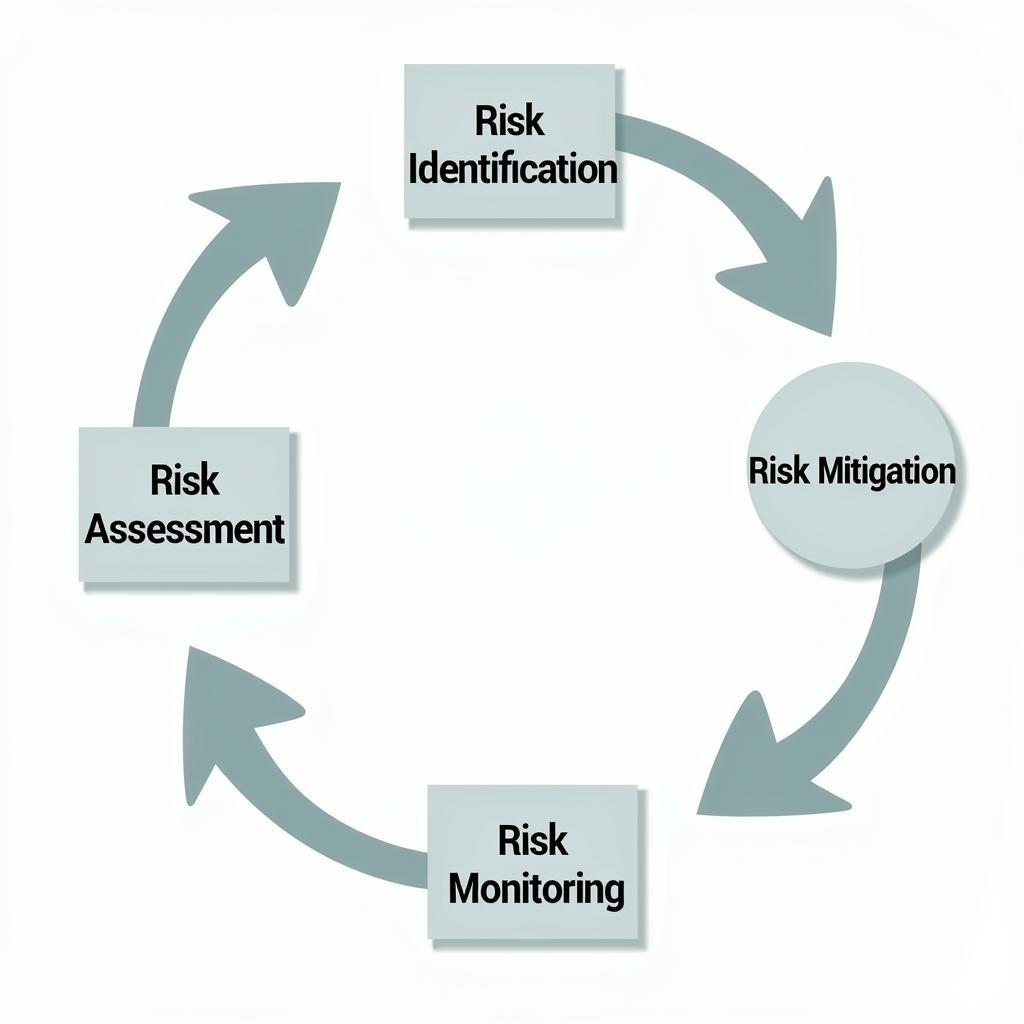 Clinical Research Risk Management Process Flowchart
Clinical Research Risk Management Process Flowchart
Understanding the Importance of Clinical Research Risk Management
Why is clinical research risk management so important? Failing to effectively manage risks can lead to serious consequences, including adverse events for participants, flawed data, regulatory sanctions, and even the termination of a study. A robust risk management plan helps protect participants’ well-being, ensures the validity of research findings, and ultimately contributes to the advancement of medical knowledge.
What are some key areas where risks typically arise in clinical research? These include protocol design, patient recruitment and selection, data collection and management, informed consent processes, and adverse event reporting. A comprehensive risk assessment should consider all these stages and potential vulnerabilities.
One crucial aspect of risk management is the development of a risk mitigation plan. This plan outlines specific strategies to address identified risks, such as implementing rigorous training programs for research staff, establishing clear procedures for data quality control, and ensuring appropriate oversight by an ethics committee.
Implementing Effective Risk Management Strategies
Effective clinical research risk management necessitates a systematic approach. This often involves establishing a dedicated risk management team, employing specialized risk management software, and integrating risk considerations into all aspects of the study design and execution.
 Risk Assessment Matrix in Clinical Research
Risk Assessment Matrix in Clinical Research
A crucial element in implementing successful risk management is appropriate training. All research personnel should be adequately trained on the study protocol, relevant regulations, and the institution’s risk management procedures. This ensures everyone is aware of their responsibilities and can effectively contribute to a safe and ethical research environment. For more information on training, check out our study start up training checklist for research staff.
Another vital component is ongoing monitoring. Regular reviews of study data, safety reports, and adherence to the protocol allow for the early identification of potential problems and the timely implementation of corrective actions. Continuous monitoring ensures that the risk management plan remains relevant and effective throughout the study’s duration. This continuous process aligns with the goals of a clinical research management course.
Best Practices in Clinical Research Risk Management
Adopting best practices in clinical research risk management can significantly enhance the safety and efficiency of clinical trials. Some key best practices include:
- Proactive risk identification: Don’t wait for problems to arise. Actively seek out potential risks from the outset and throughout the study.
- Comprehensive risk assessment: Evaluate both the likelihood and potential impact of each identified risk.
- Detailed risk mitigation plans: Develop clear, actionable strategies to address each identified risk.
- Continuous monitoring and evaluation: Regularly review and update the risk management plan based on emerging data and experience.
“Proactive risk management isn’t just a best practice; it’s an ethical imperative in clinical research,” says Dr. Emily Carter, a leading expert in clinical trial methodology. “Protecting the well-being of participants and ensuring the integrity of research data must always be our top priority.”
“Effective risk management requires a collaborative approach,” adds Dr. James Miller, a seasoned clinical research professional. “Open communication and information sharing among all stakeholders are essential for identifying and mitigating potential risks.” If you are considering a career as a clinical research manager, you may want to explore options for equity research analyst certification. There are also valuable resources on management controls that are built in to a research study.
Conclusion
Clinical research risk management is not a one-time activity but a continuous process that requires ongoing attention and adaptation. By implementing a robust risk management plan and following best practices, we can enhance patient safety, ensure data integrity, and increase the likelihood of successful clinical trials. This contributes not only to the advancement of medical knowledge but also to building public trust in the research enterprise. Clinical research risk management is vital for ethical and successful research.
FAQs
Are you interested in research topics for nurses?
Need support? Contact us 24/7: Phone: 0904826292, Email: research@gmail.com. Visit us at: No. 31, Alley 142/7, P. Phú Viên, Bồ Đề, Long Biên, Hà Nội, Việt Nam.