Firma Clinical Research plays a crucial role in advancing medical knowledge and developing new treatments. This complex process involves rigorous testing and evaluation to ensure the safety and efficacy of new drugs, devices, and therapies. Understanding the intricacies of firma clinical research is vital for both healthcare professionals and individuals interested in participating in clinical trials.
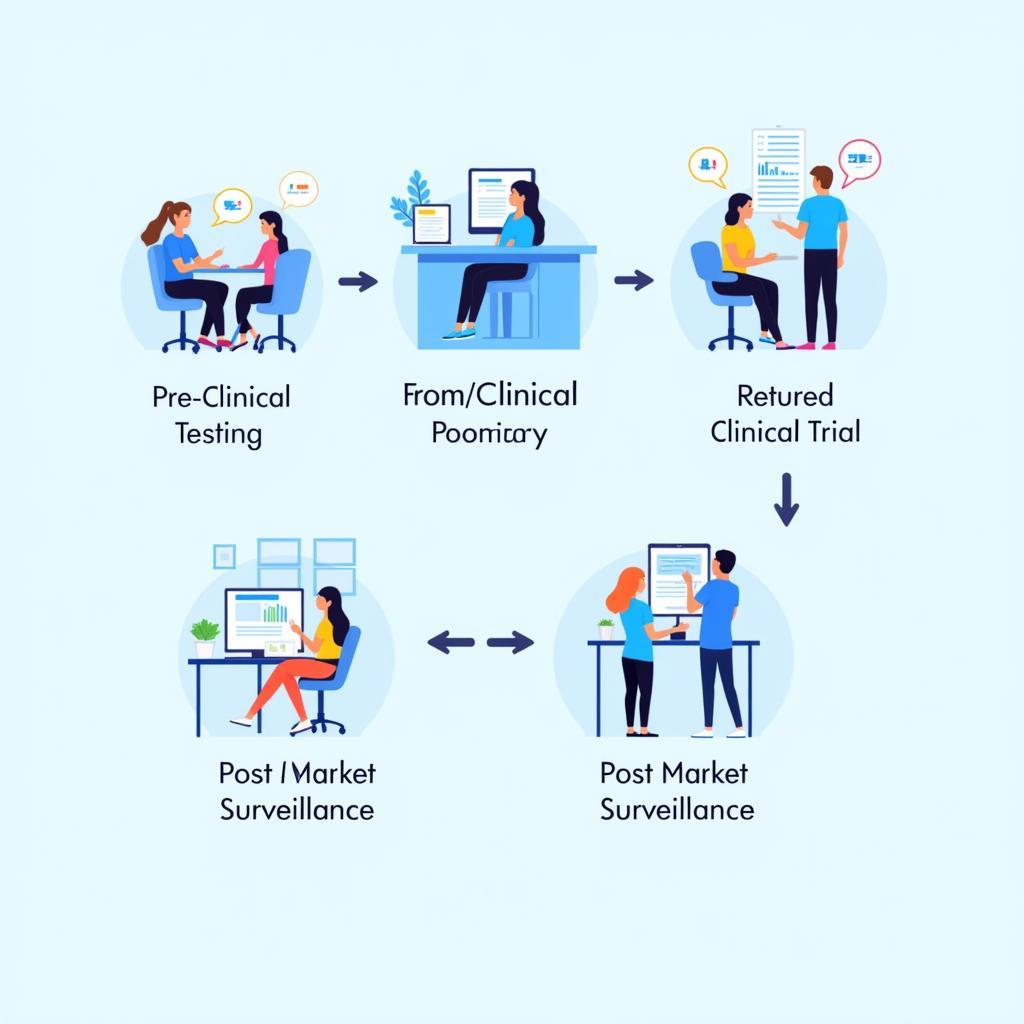 The Clinical Research Process
The Clinical Research Process
What is Firma Clinical Research?
Clinical research, often conducted by “firmas” or companies, is a systematic investigation in human beings designed to contribute to medical knowledge. It involves careful observation and data analysis to determine the effects of a particular intervention. These interventions can include new medications, surgical procedures, diagnostic tools, or behavioral therapies. The ultimate goal is to improve patient care and enhance public health.
What makes clinical research, particularly within a “firma” or company setting, distinct is the focus on developing commercially viable treatments and technologies. While academic and government-funded research plays a vital role in basic scientific discovery, firma clinical research often bridges the gap between scientific advancement and practical application.
Why is Firma Clinical Research Important?
Firma clinical research is essential for bringing new and improved treatments to the market. Without it, medical progress would stagnate, and patients would be deprived of potentially life-saving therapies. Through research management and oversight by research supervisors, the clinical research process is constantly being refined. Furthermore, firma clinical research provides valuable data that can inform public health policies and guidelines.
How Does Firma Clinical Research Work?
The process of firma clinical research typically involves several phases: pre-clinical testing, where researchers study the intervention in the lab and in animals; Phase I trials, which evaluate the safety of the intervention in a small group of healthy volunteers; Phase II trials, which assess the efficacy of the intervention in a larger group of patients with the target condition; and Phase III trials, which compare the new intervention to existing treatments in a large, multi-center study. Post-market surveillance continues to monitor the long-term effects of the intervention. Using the best ai tool for research can significantly streamline these phases.
Navigating the Challenges of Firma Clinical Research
Firma clinical research is not without its challenges. One of the major hurdles is recruiting and retaining participants. Many clinical trials struggle to find enough volunteers who meet the specific criteria. Additionally, firma clinical research faces ethical considerations related to informed consent, patient safety, and data privacy.
What is the Outcome of Research?
The what is the outcome of research question, particularly within the context of firma clinical research, often revolves around tangible results. These include the development of new drugs and devices, improved treatment protocols, and a deeper understanding of diseases and their mechanisms.
Quote from Dr. Amelia Hernandez, Chief Medical Officer at BioNova Pharmaceuticals: “Firma clinical research is the engine of medical innovation. It’s how we turn promising discoveries into tangible treatments that can improve and save lives.”
The Future of Firma Clinical Research
The field of firma clinical research is constantly evolving, incorporating new technologies and methodologies. Personalized medicine, which tailors treatments to individual patients based on their genetic makeup and other factors, is one of the most exciting developments in the field. Qualitative research nursing articles contribute to our understanding of this nuanced area.
Quote from Dr. David Lee, Director of Clinical Research at MedTech Innovations: “The future of firma clinical research lies in personalized medicine and the use of big data to accelerate the development of more effective and targeted therapies.”
In conclusion, firma clinical research is a critical component of medical advancement. By understanding the process and its complexities, we can appreciate its vital role in improving patient care and fostering a healthier future.
FAQ
- What is the difference between firma clinical research and academic research?
- How can I participate in a clinical trial?
- What are the risks and benefits of participating in a clinical trial?
- How are clinical trials regulated?
- What is informed consent in the context of clinical research?
- How long does a clinical trial typically last?
- What happens after a clinical trial is completed?
Need further assistance? Contact us at:
Phone: 0904826292
Email: research@gmail.com
Address: No. 31, Alley 142/7, P. Phú Viên, Bồ Đề, Long Biên, Hà Nội, Việt Nam.
Our customer service team is available 24/7.