Statistics plays a crucial role in every stage of biopharmaceutical research, from drug discovery and development to clinical trials and regulatory approval. It provides the framework for designing studies, analyzing data, and drawing meaningful conclusions. Understanding statistical concepts is essential for researchers, clinicians, and regulators involved in this complex field.
Why are Statistics Important in Biopharmaceutical Research?
Statistical methods are used to evaluate the safety and efficacy of new drugs and therapies. They help researchers determine the appropriate dosage, identify potential side effects, and assess the overall benefit-risk profile of a treatment. Without robust statistical analysis, it would be impossible to make informed decisions about the development and use of biopharmaceuticals.
Designing Effective Studies with Statistical Power
One of the key applications of statistics is in the design of clinical trials. Researchers use statistical power calculations to determine the optimal sample size needed to detect a clinically meaningful difference between treatment groups. This ensures that the study has enough participants to provide reliable and statistically significant results. Factors like effect size, significance level, and variability are carefully considered during the planning phase.
Analyzing Data and Drawing Meaningful Conclusions
Once data is collected, statistical methods are used to analyze and interpret the results. Researchers use a variety of statistical tests, such as t-tests, ANOVA, and regression analysis, to compare treatment groups and identify statistically significant differences. These analyses help researchers understand the effectiveness of a treatment and identify any potential safety concerns.
“Accurate data analysis is the cornerstone of credible biopharmaceutical research,” states Dr. Emily Carter, a leading biostatistician. “Without rigorous statistical methods, we risk drawing inaccurate conclusions that could have serious implications for patient health.”
Ensuring Regulatory Compliance and Drug Approval
Statistical analysis is also critical for regulatory compliance. Regulatory agencies, such as the FDA, require rigorous statistical evidence to demonstrate the safety and efficacy of new drugs before they can be approved for market. Biopharmaceutical companies must submit detailed statistical reports as part of their regulatory submissions.
Key Statistical Concepts in Biopharmaceutical Research
Several key statistical concepts are essential for understanding biopharmaceutical research. These include hypothesis testing, p-values, confidence intervals, and statistical significance.
Hypothesis Testing and P-values
Hypothesis testing is a fundamental statistical method used to evaluate research questions. Researchers formulate a null hypothesis, which states that there is no difference between treatment groups, and an alternative hypothesis, which states that there is a difference. Statistical tests are then used to determine whether the data supports the null or alternative hypothesis. The p-value is a measure of the strength of evidence against the null hypothesis. A small p-value (typically less than 0.05) indicates strong evidence against the null hypothesis and suggests that there is a statistically significant difference between treatment groups.
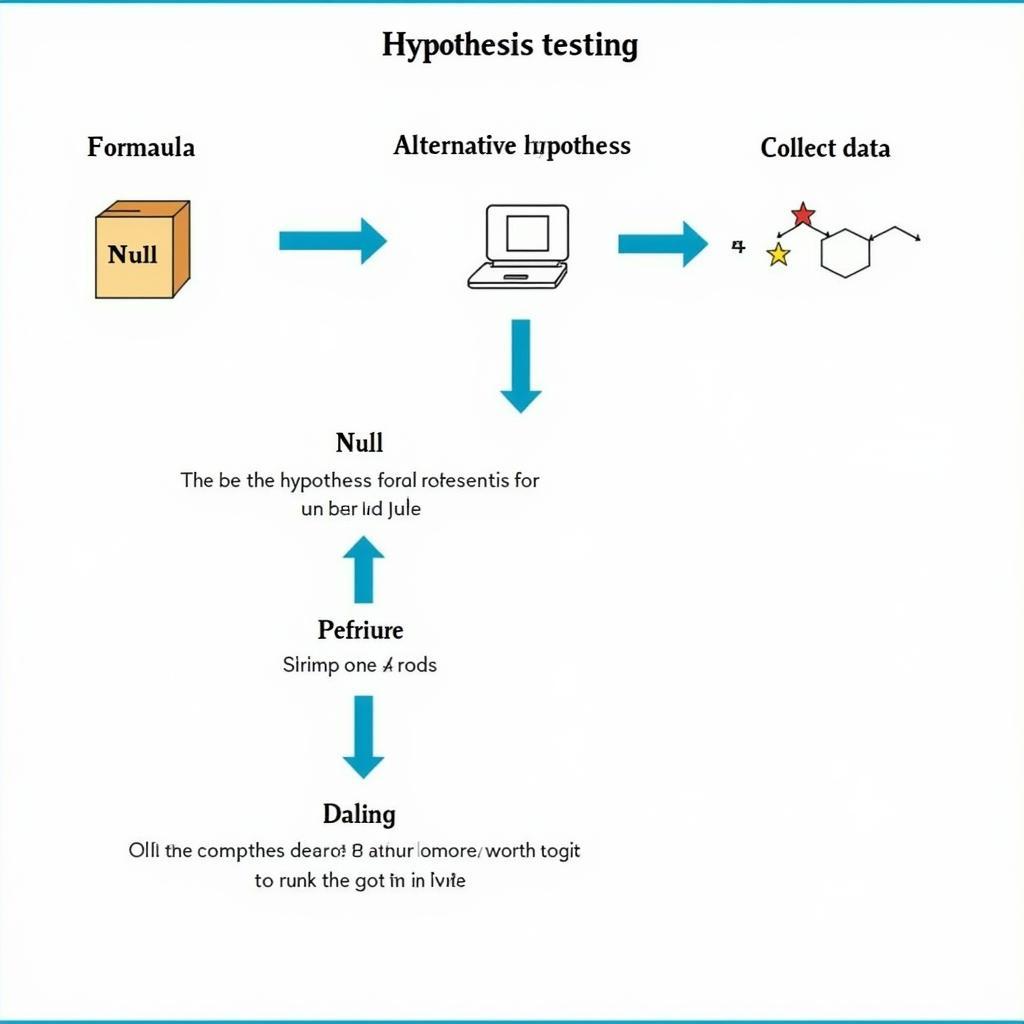 Hypothesis Testing and P-value in Biopharmaceutical Research
Hypothesis Testing and P-value in Biopharmaceutical Research
Confidence Intervals and Statistical Significance
Confidence intervals provide a range of values within which the true population parameter is likely to fall. For example, a 95% confidence interval for the mean difference between two treatment groups would indicate that there is a 95% probability that the true mean difference lies within that range. Statistical significance is a measure of how likely it is that an observed effect is not due to chance. A result is considered statistically significant if the p-value is less than a pre-defined threshold, typically 0.05.
“Understanding confidence intervals is crucial for interpreting research findings,” explains Dr. David Miller, a senior clinical researcher. “They provide a more nuanced understanding of the uncertainty associated with statistical estimates.”
Conclusion
Statistics In Biopharmaceutical Research is essential for ensuring the safety and efficacy of new drugs and therapies. By understanding statistical concepts and methods, researchers can design robust studies, analyze data accurately, and draw meaningful conclusions that can ultimately improve patient outcomes. Utilizing proper statistical analysis throughout the research process is vital for advancing biopharmaceutical knowledge and bringing effective treatments to market.
FAQ
- What software is commonly used for statistical analysis in biopharmaceutical research?
- How are statistical methods used to address bias in clinical trials?
- What are some of the challenges associated with statistical analysis in biopharmaceutical research?
- How are statistical methods used to analyze survival data in clinical trials?
- What is the role of Bayesian statistics in biopharmaceutical research?
- How can statistical literacy be improved among researchers and clinicians?
- What are some emerging trends in statistical methodology for biopharmaceutical research?
Need help with your research? Contact us at Phone: 0904826292, Email: research@gmail.com or visit our office at No. 31, Alley 142/7, P. Phú Viên, Bồ Đề, Long Biên, Hà Nội, Việt Nam. We have a 24/7 customer support team.