Clinical Research Fastrack Reviews have become increasingly important in the world of medicine, offering a streamlined approach to getting new treatments and therapies to patients faster. This guide will delve into the intricacies of clinical research fastrack reviews, providing valuable insights for both patients and researchers alike.
Understanding the Need for Speed: Why Fastrack Reviews Matter
Traditional clinical research pathways often involve lengthy timelines that can delay the availability of potentially life-saving treatments. Fastrack reviews aim to address this challenge by expediting the evaluation and approval process for promising new therapies, particularly those targeting serious or life-threatening conditions.
Inside the World of Fastrack Reviews: Key Mechanisms and Processes
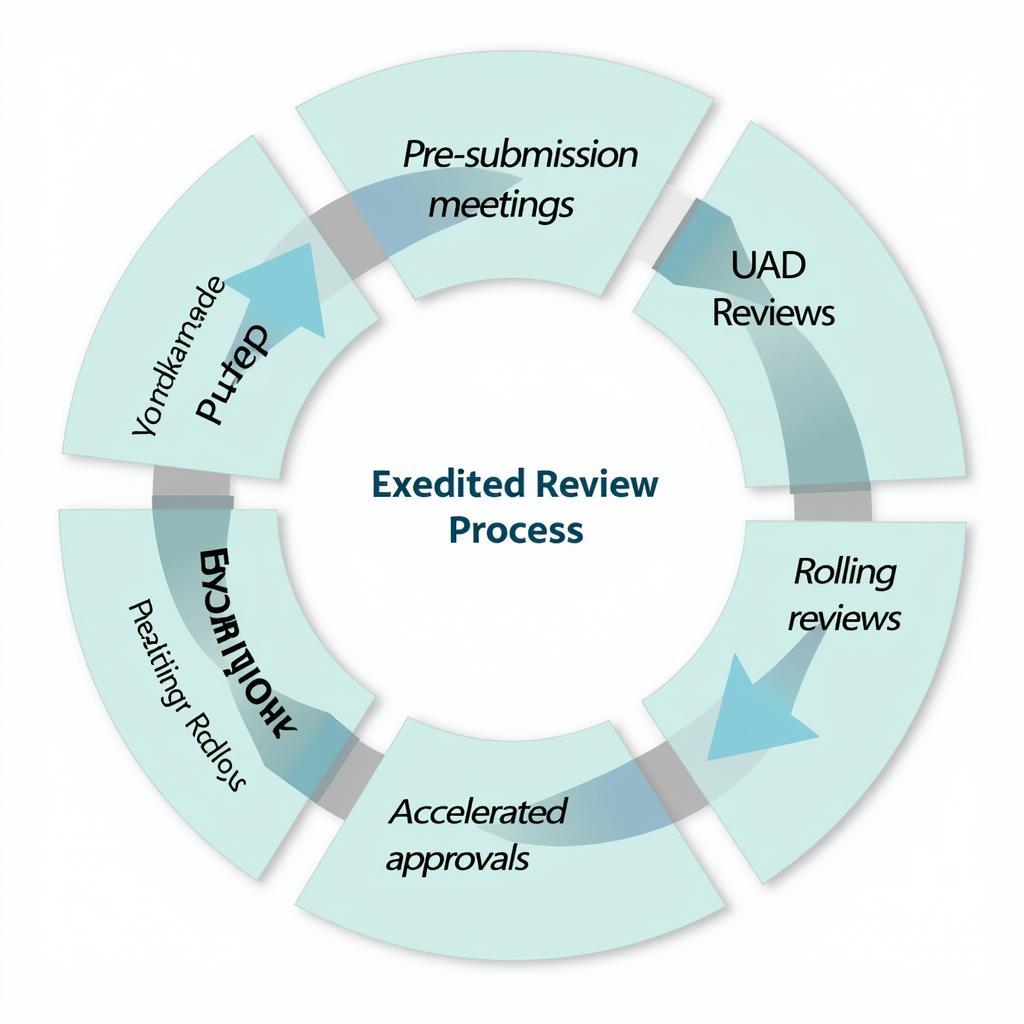 clinical-trial-fastrack-review-process-flowchart
clinical-trial-fastrack-review-process-flowchart
Several mechanisms contribute to the efficiency of fastrack reviews:
- Priority Review: Regulatory bodies like the FDA prioritize applications for therapies addressing unmet medical needs, potentially shortening review times.
- Accelerated Approval: This pathway allows for earlier approval based on surrogate endpoints, which are markers that are reasonably likely to predict clinical benefit.
- Breakthrough Therapy Designation: This designation is granted to therapies demonstrating substantial improvement over existing treatments for serious conditions.
- Rolling Review: This approach allows researchers to submit sections of their application as they are completed, rather than waiting for the entire document to be finalized.
Benefits and Challenges of Fastrack Reviews: A Balanced Perspective
The advantages of fastrack reviews are significant, primarily the potential to bring innovative therapies to patients sooner. This can translate to improved outcomes, particularly for individuals facing critical illnesses.
 patients-benefitting-from-fastrack-review-of-new-treatment
patients-benefitting-from-fastrack-review-of-new-treatment
However, it’s important to acknowledge the potential challenges associated with expedited reviews. These may include:
- Limited Long-Term Data: Fastrack approvals often rely on shorter-term data, and the long-term safety and efficacy of a therapy might not be fully understood at the time of approval.
- Risk-Benefit Considerations: Balancing the need for rapid access with the potential risks of a new therapy requires careful evaluation.
“While fastrack reviews offer immense promise, it’s crucial to maintain rigorous scientific standards throughout the process to ensure patient safety remains paramount,” notes Dr. Emily Carter, a leading clinical research expert.
The Future of Fastrack Reviews: Innovation and Patient-Centric Approaches
The landscape of clinical research fastrack reviews is continuously evolving. New methodologies and technologies are being explored to further enhance efficiency and decision-making.
Moreover, there is growing emphasis on incorporating patient perspectives into the review process, recognizing that patients are not merely recipients of treatment but active participants in healthcare decisions.
Conclusion
Clinical research fastrack reviews represent a significant advancement in medical innovation, offering a pathway for accelerating access to promising new therapies. By understanding the mechanisms, benefits, and challenges associated with fastrack reviews, we can navigate this evolving landscape and continue to strive towards better and faster treatment options for patients in need.
FAQ
1. What types of therapies are eligible for fastrack review?
Therapies addressing serious or life-threatening conditions with the potential to offer significant advantages over existing treatments are typically considered.
2. Who decides if a therapy qualifies for fastrack review?
Regulatory bodies like the FDA have specific criteria and guidelines for determining eligibility.
3. Are fastrack reviews less rigorous than traditional reviews?
No, the same high standards of scientific evidence and evaluation apply, but the process is expedited.
4. Can patients advocate for fastrack review of a particular therapy?
Patients and advocacy groups can raise awareness and engage in dialogue with regulatory bodies regarding promising treatments.
5. Where can I find more information about specific therapies under fastrack review?
Regulatory agency websites and reputable medical journals often provide updates on ongoing clinical trials and approvals.
Still have questions?
Contact us today for personalized support.
Phone: 0904826292
Email: research@gmail.com
Address: No. 31, Alley 142/7, P. Phú Viên, Bồ Đề, Long Biên, Hà Nội, Việt Nam
Our dedicated team is available 24/7 to assist you.