Ophthalmology Contract Research Organizations (CROs) play a critical role in bringing new eye care treatments and technologies to market. These specialized organizations provide a wide range of services to pharmaceutical, biotechnology, and medical device companies looking to conduct clinical trials and research in the field of ophthalmology.
What is an Ophthalmology CRO?
An ophthalmology CRO is a company that provides support to the pharmaceutical, biotechnology, and medical device industries in the form of research services outsourced on a contract basis. They specialize in conducting clinical trials and other research studies related to the diagnosis, treatment, and prevention of eye diseases.
Why are Ophthalmology CROs Important?
Ophthalmology research is highly specialized and requires expertise in areas such as ocular imaging, visual function testing, and ophthalmic surgical procedures. CROs with a specific focus on ophthalmology have the knowledge, experience, and resources to design and execute complex clinical trials in this field.
“Partnering with an experienced ophthalmology CRO is crucial for navigating the unique regulatory landscape and ensuring high-quality data collection in eye care research,” says Dr. Emily Carter, a leading ophthalmologist and clinical trial expert.
Key Services Offered by Ophthalmology CROs
Ophthalmology CROs provide a comprehensive range of services, including:
- Clinical Trial Design and Planning: Developing study protocols, determining endpoints, and creating case report forms specifically for ophthalmic studies.
- Site Selection and Patient Recruitment: Identifying and qualifying clinical trial sites with experienced ophthalmologists and recruiting eligible patients.
- Data Management and Statistical Analysis: Collecting, managing, and analyzing clinical trial data using specialized software and statistical methods.
- Regulatory Affairs: Preparing and submitting regulatory documents to ethics committees and regulatory agencies.
- Medical Writing: Creating clinical study reports, manuscripts for publication, and other essential documents.
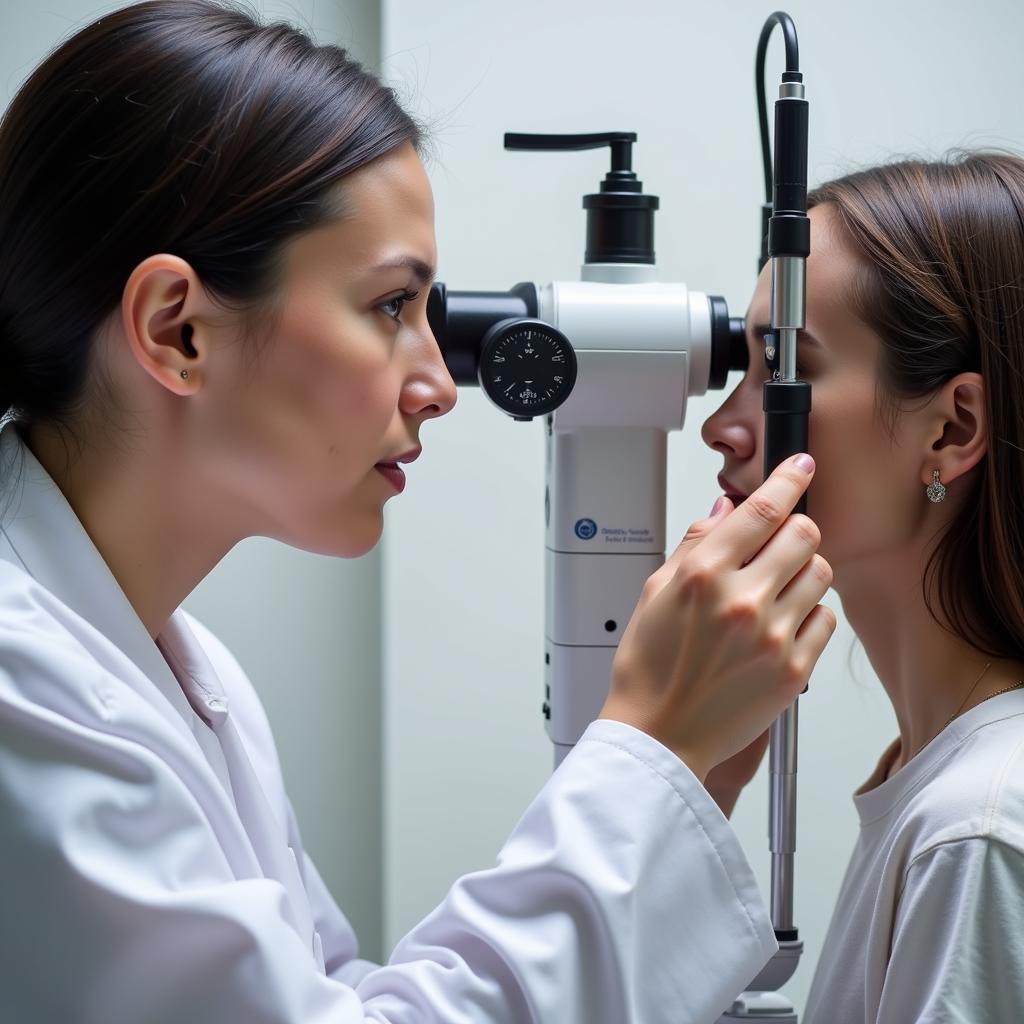 Conducting an ophthalmology clinical trial
Conducting an ophthalmology clinical trial
How to Choose the Right Ophthalmology CRO
Selecting the right ophthalmology CRO is essential for the success of your clinical trial. Here are key factors to consider:
- Experience and Expertise: Look for a CRO with a proven track record in ophthalmology research, a team of experienced professionals, and a deep understanding of eye diseases.
- Therapeutic Area Specialization: Choose a CRO specializing in your specific therapeutic area of interest, such as retina, glaucoma, or cornea.
- Global Reach: If your trial requires a global presence, consider a CRO with a network of sites and resources in multiple countries.
- Technology and Infrastructure: Evaluate the CRO’s data management systems, electronic data capture (EDC) capabilities, and other technological infrastructure.
- Communication and Collaboration: Effective communication and collaboration are essential for a successful partnership. Choose a CRO that is responsive, transparent, and committed to open dialogue.
Benefits of Working with an Ophthalmology CRO
Partnering with an ophthalmology CRO offers numerous benefits, including:
- Accelerated Timelines: CROs can help expedite the clinical trial process, from study startup to data analysis and reporting.
- Cost-Effectiveness: Outsourcing research activities to a CRO can be more cost-effective than building in-house capabilities.
- Access to Expertise: CROs provide access to a team of highly skilled professionals with specialized knowledge in ophthalmology research.
- Reduced Risk: CROs have established processes and systems in place to mitigate risk and ensure compliance with regulatory requirements.
- High-Quality Data: CROs are equipped to collect, manage, and analyze clinical trial data with accuracy and precision.
 Analyzing ophthalmology research data
Analyzing ophthalmology research data
Conclusion
Ophthalmology CROs are indispensable partners in advancing eye care research and bringing innovative treatments to patients worldwide. By leveraging their specialized expertise, resources, and commitment to quality, pharmaceutical and biotechnology companies can enhance their clinical trial efforts and improve patient outcomes in the field of ophthalmology.